1 3 5 Hexatriene Mo Diagram
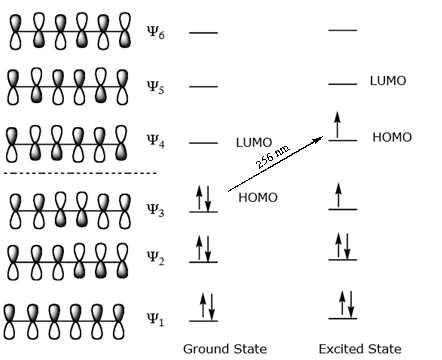
In molecules with extended pi systems the homo lumo energy gap becomes so small that absorption occurs in the visible rather then the uv region of the electromagnetic spectrum.
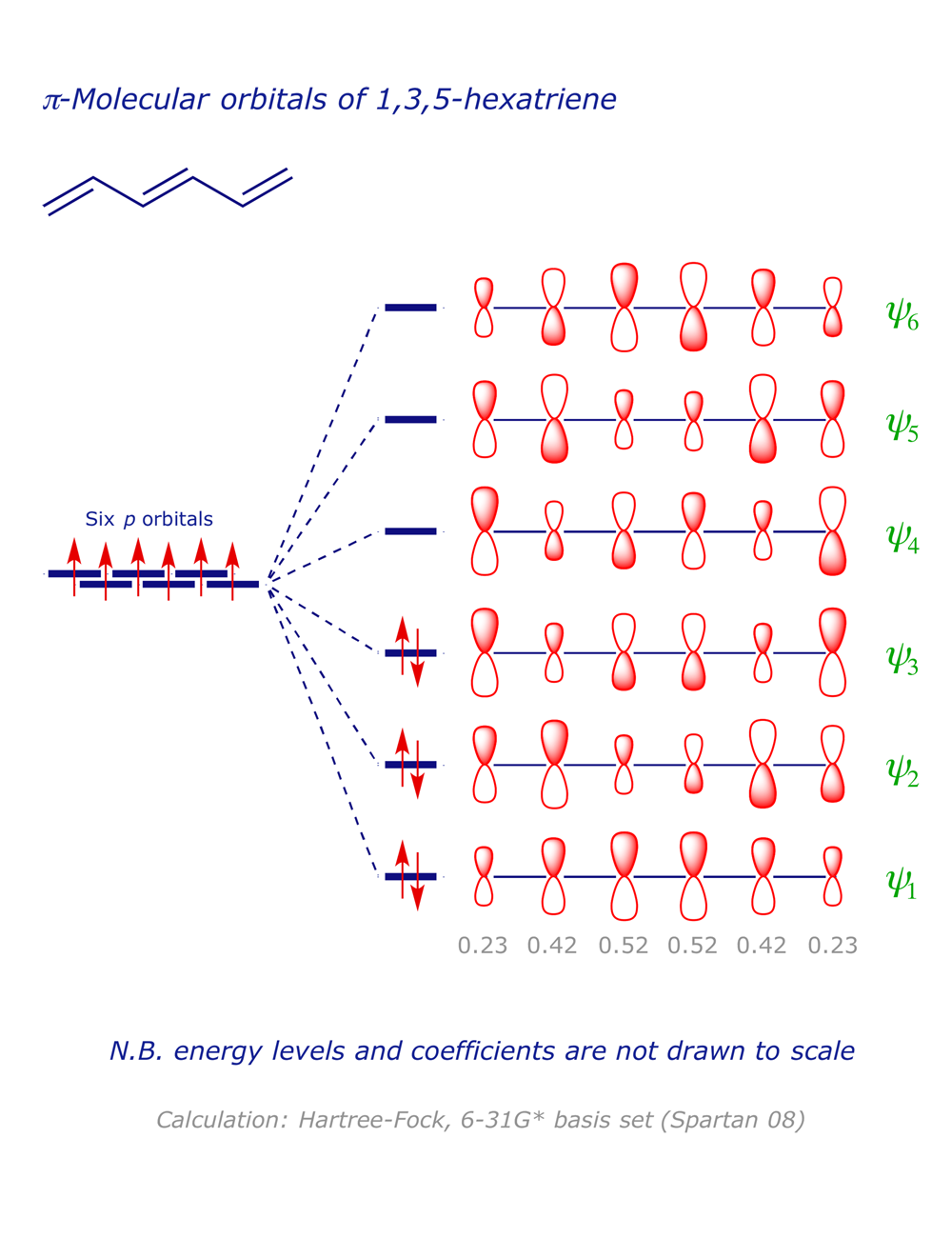
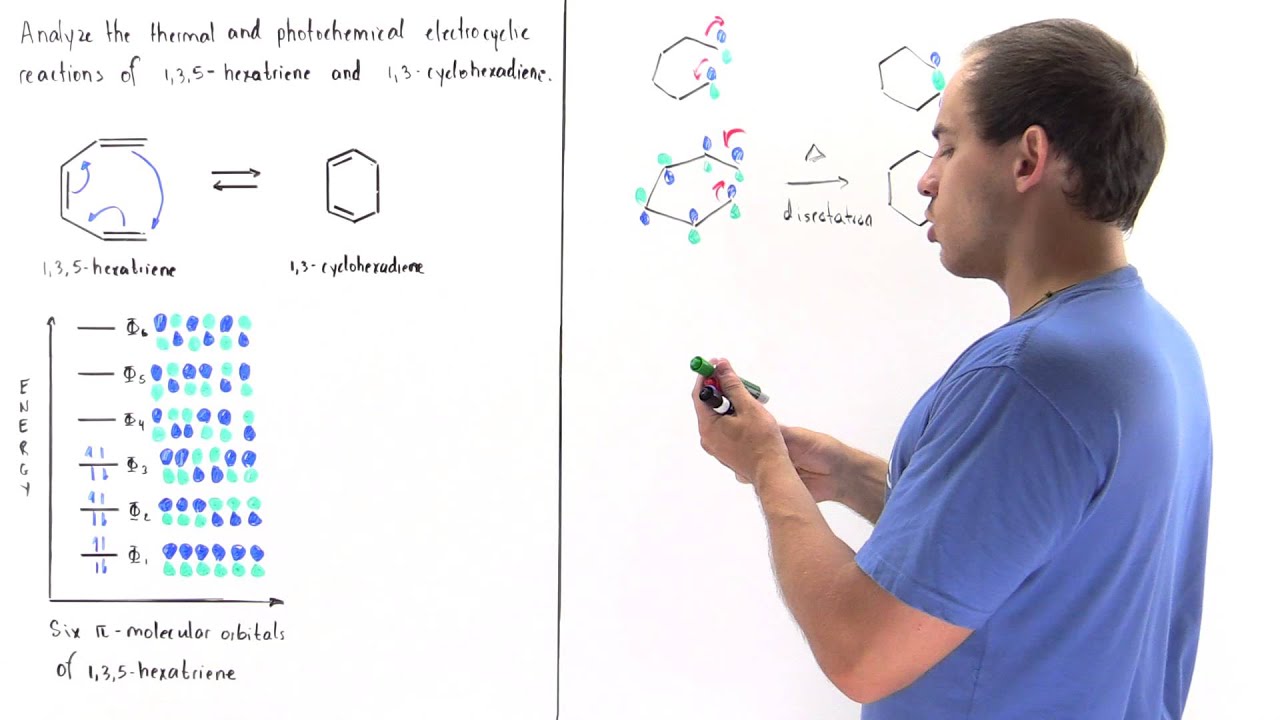
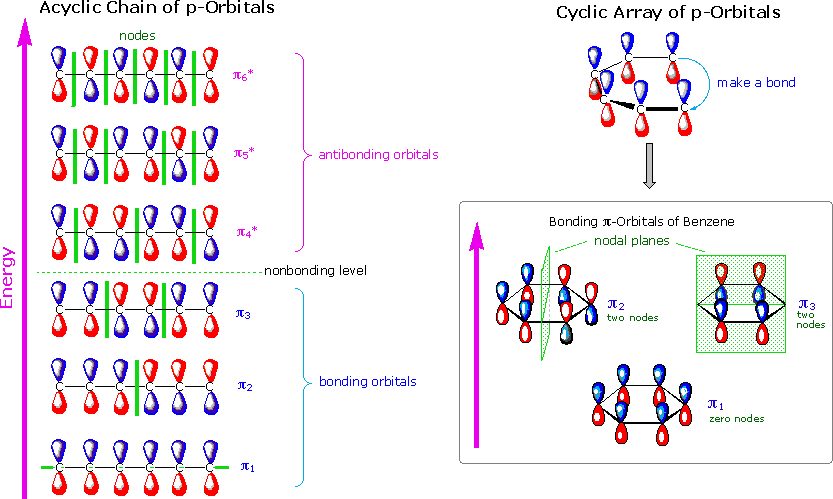
1 3 5 hexatriene mo diagram. 6 π molecular orbitals of hexatriene five nodes four nodes three nodes two nodes one node zero nodes 6 ao s 6 mo s six p orbitals 6 conjugation. The absorbance due to the π π transition in 1 3 5 hexatriene for example occurs at 258 nm corresponding to a δ e of 111 kcal mol. To illustrate how we apply hückel in practice let s work out the energy of benzene as an example. As in the previous examples use the aufbau principle hund s rule and the pauli exclusion principle to determine the ground state electron configuration.
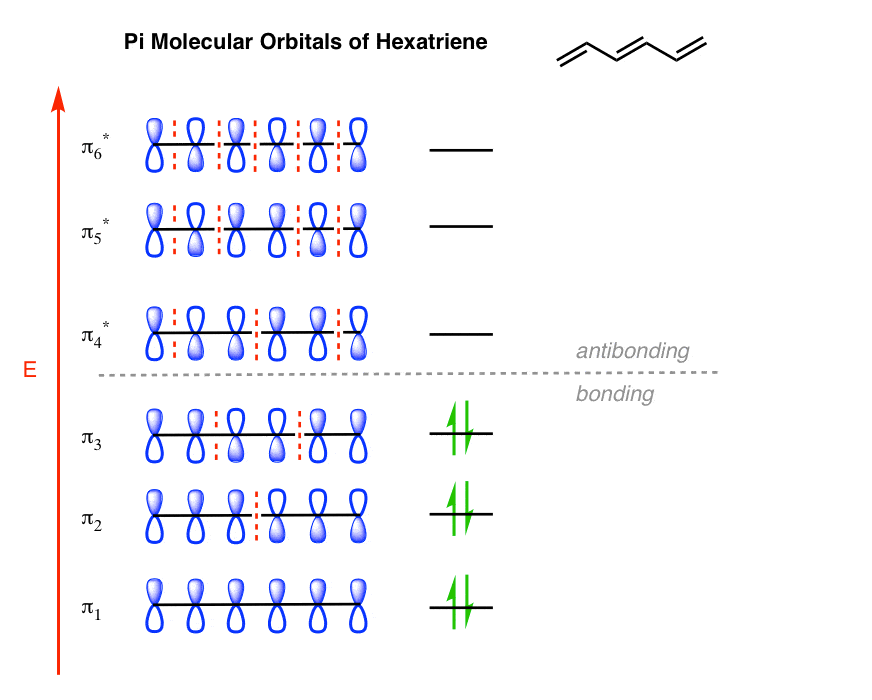
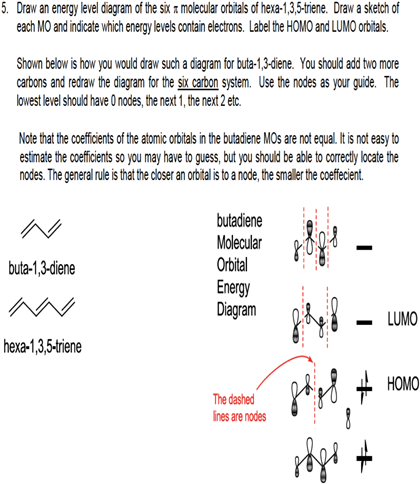
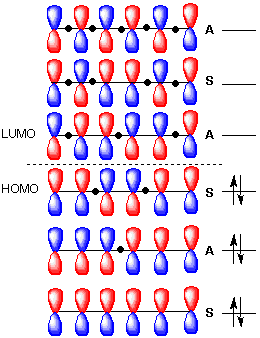
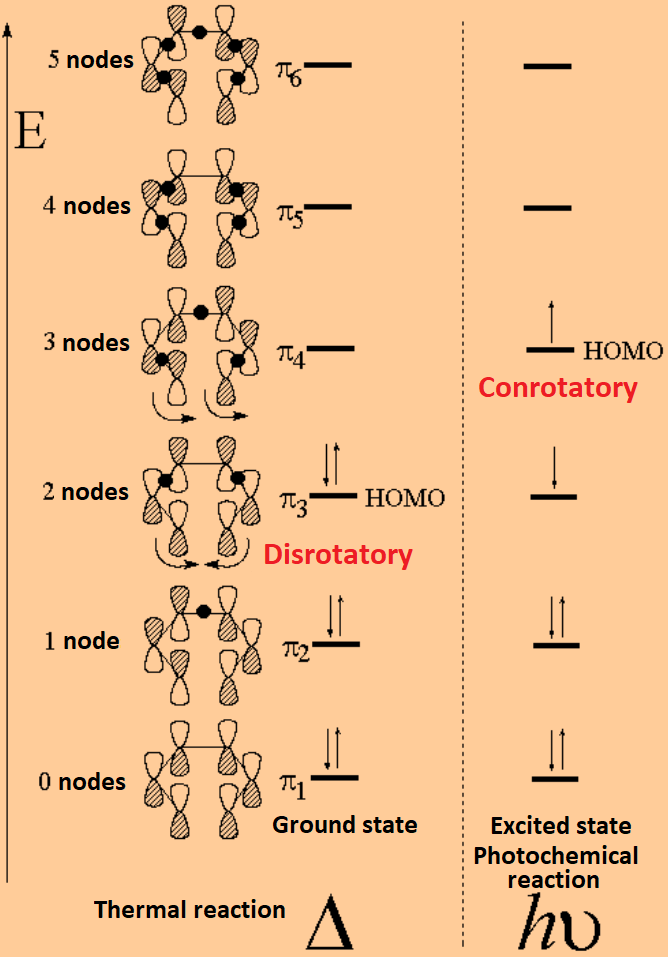
Note all lobes for each carbon bonding interactions the number of nodes in the mo where wave function changes and classify lumo homo. Draw the molecular orbital diagram of 1 3 5 hexatriene including pictures of each of the molecular orbitals showing the phase of each of the p orbitals. I 1 where ei are the mo eigenvalues determined in the third step. 1 2 5 3 6 4 1 each of the mos is a linear combination of 6 pz orbitals cµ 1 µ c2 6 cµ ψµ cµ p z i cµ 3 i µ i 1 c4.
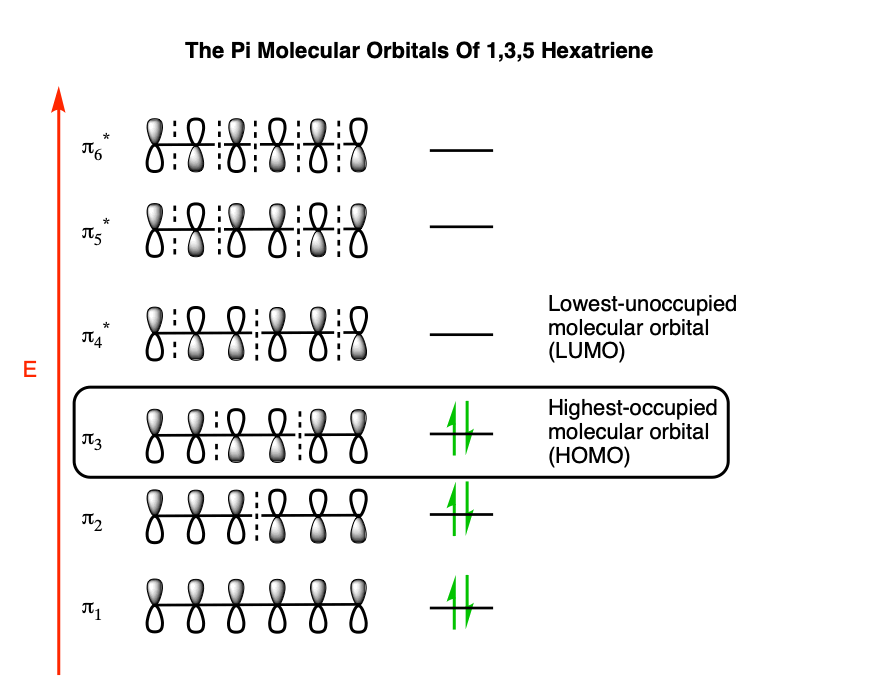
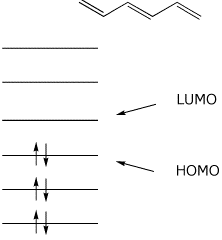
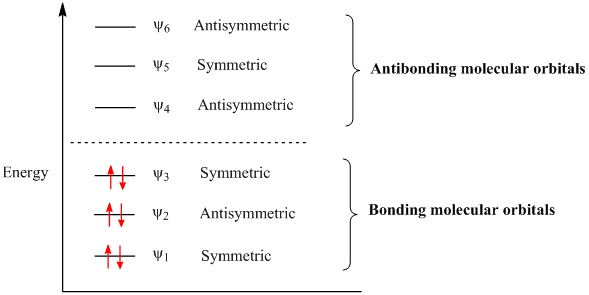
Want chad s organic chem. Lebel each of the molecular orbitals as bonding and anti bonding. The orbitals are arranged in the following table in order of increasing energy. ψ 1 ψ 2 and ψ 3 are bonding molecular orbitals and are occupied in the ground state with ψ 3 being the highest occupied molecular orbital homo.
Mos of 1 3 5 hexatriene new resizable window. ψ 4 ψ 5 and ψ 6 are antibonding molecular orbitals and are unoccupied in the ground state with ψ 4 being the lowest unoccupied molecular orbital lumo. Molecular orbital energy level diagram of 1 3 5 7 octatetraene. Fill in the electrons in the mo diagram.
Cyclic conjugated organic compounds such as benzene that exhibit special stability due to resonance delocalization of π electrons. Series of overlapping p orbitals aromaticity.