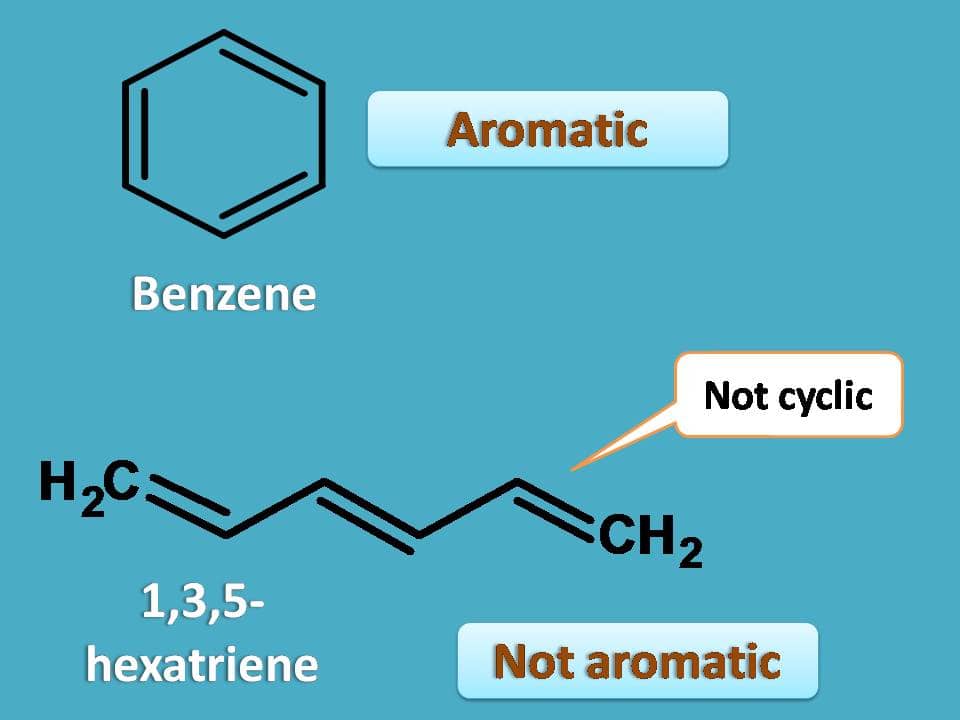
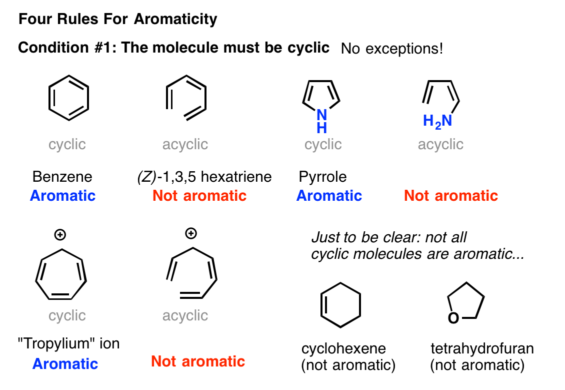
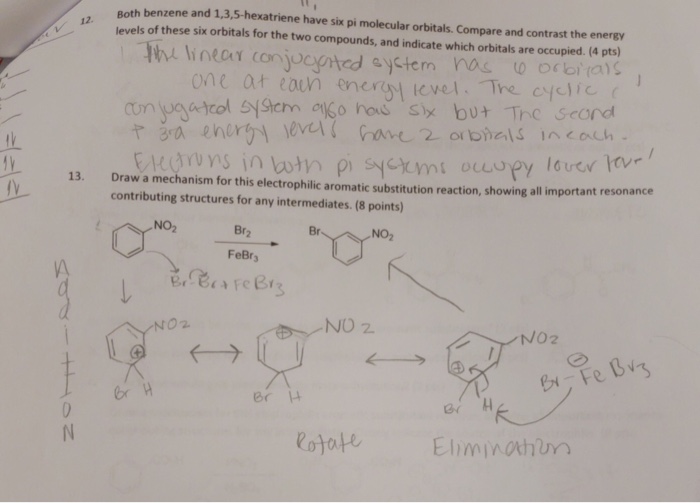
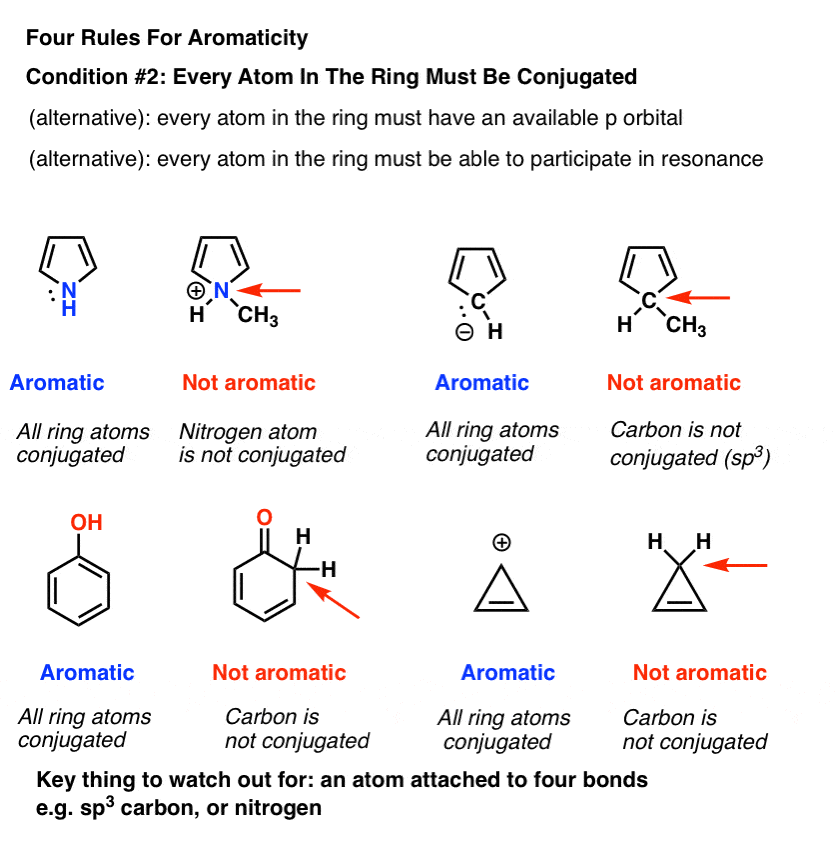
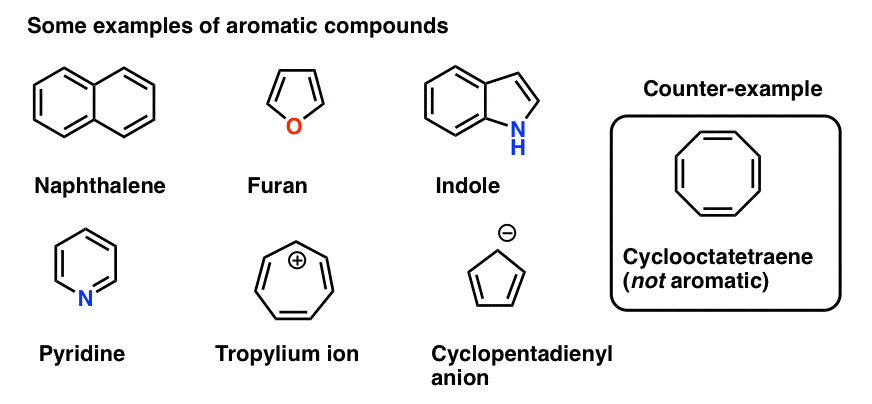
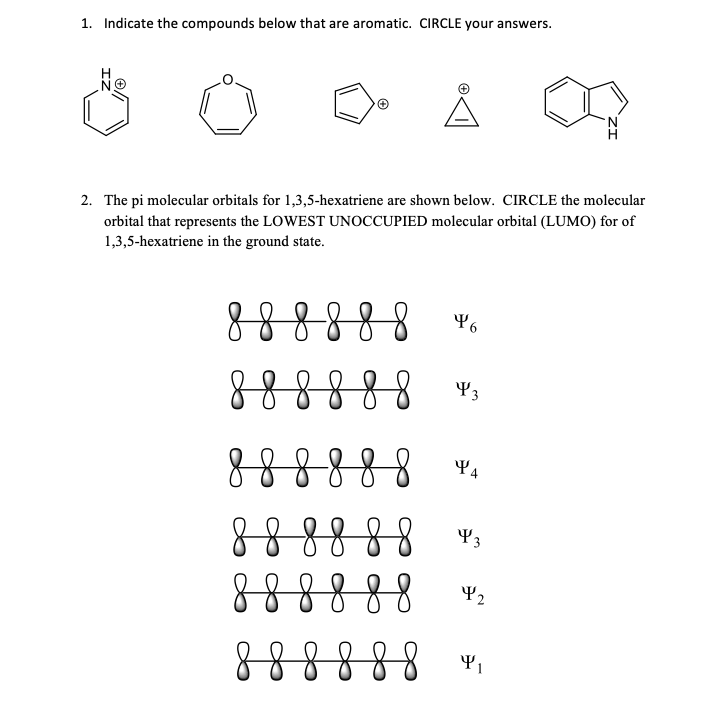
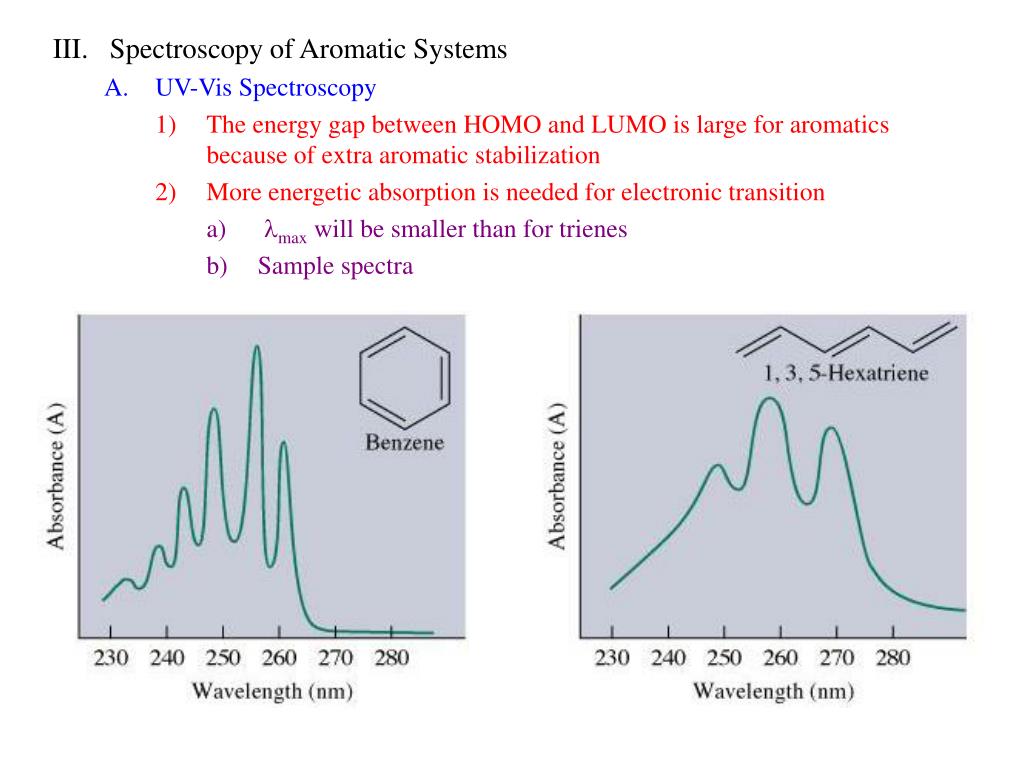
1 3 5 Hexatriene Aromatic
1 3 5 hexatriene z c6h8 cid 5367415 structure chemical names physical and chemical properties classification patents literature biological activities.
1 3 5 hexatriene aromatic. 4 related records expand this section. 1 structures expand this section. 10 points a particular substituent x is identified as having the following partial rate factors for electrophilic aromatic bromination. For example benzene is more stable than 1 3 5 hexatriene.
3 1 3 dim ethylbnz meta xylene m xylene co2h cl 4 ch orbenziad p ar chl obenzid p chl orbenziad cl 10. For cyclobutadiene when you draw this diagram out it has top to bottom. For example 1 3 5 hexatriene is comparatively unstabler than the structure of benzene. 3 chemical and physical properties expand this section.
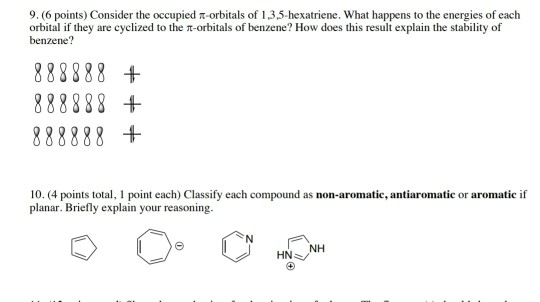
B draw approximate molecular orbital pictures of the homo and lumo of 1 3 5 hexatriene indicating orbital symmetries and the presence of any nodal planes. Why is 1 3 5 hexatriene non aromatic. For example cyclobutadiene is less stable than butadiene. Aromatic h s strongly deshielded by ring and absorb between δ 6 5 and δ 8 0 peak pattern is characteristic positions of substituents 30 c 8h 9br c 9h 12 2h 2h 2h 3h 4h 2h 3h 3h.
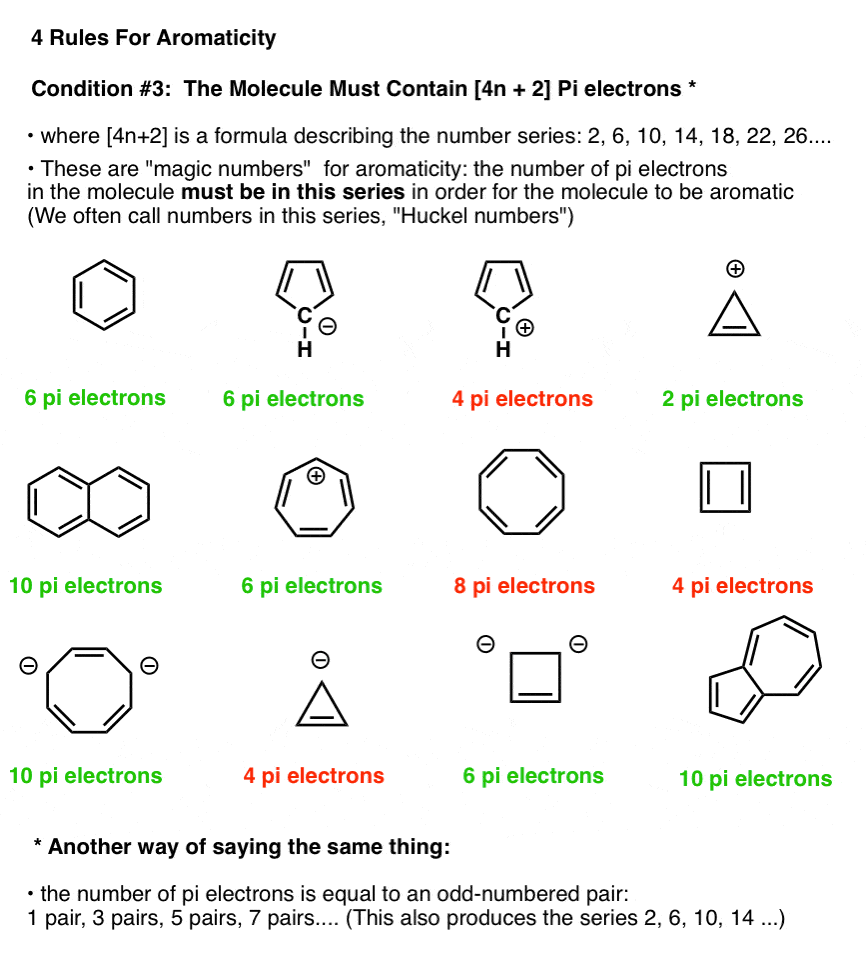
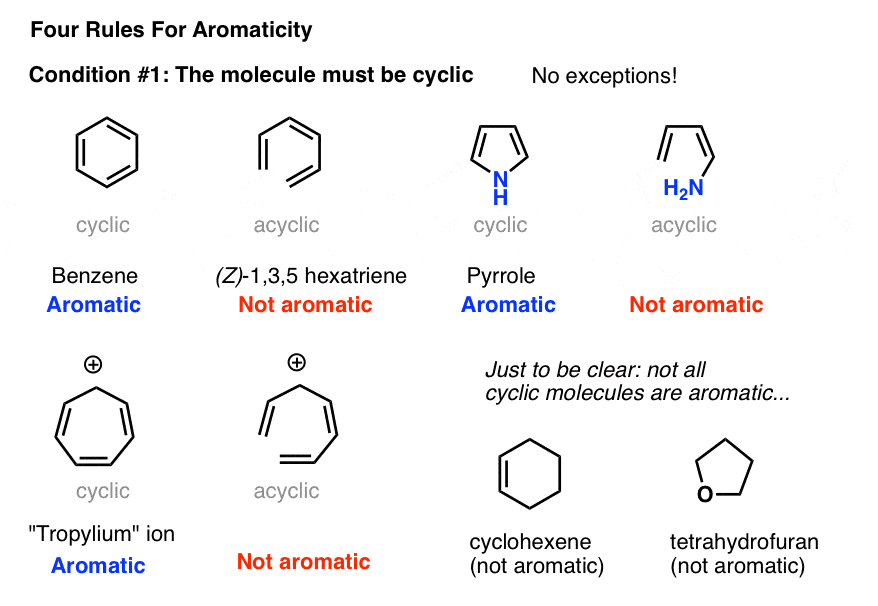
Compared to this the anti aromatic compound is one that refers to the first three criteria but delocalized pi electrons over the ring increase the electronic energy. The number of nodes are 0 1 1 2 2 and 3 respectively. An antiaromatic compound is less stable than its open chain counterpart. It can be easily visualised by comparing relative stability and chemical properties of these two compounds.
So conjugation is not only the factor responsible for aromaticity but still another factor plays important role. The aromatic structures are more stable than their open chain counterparts. In a half liter flask 5 0g pure pcl5 was introduced and equilibrium was allowed to establish. Undoubtedly it is not aromatic even it has conjugated double bonds.