1 2 And 1 4 Addition Reaction
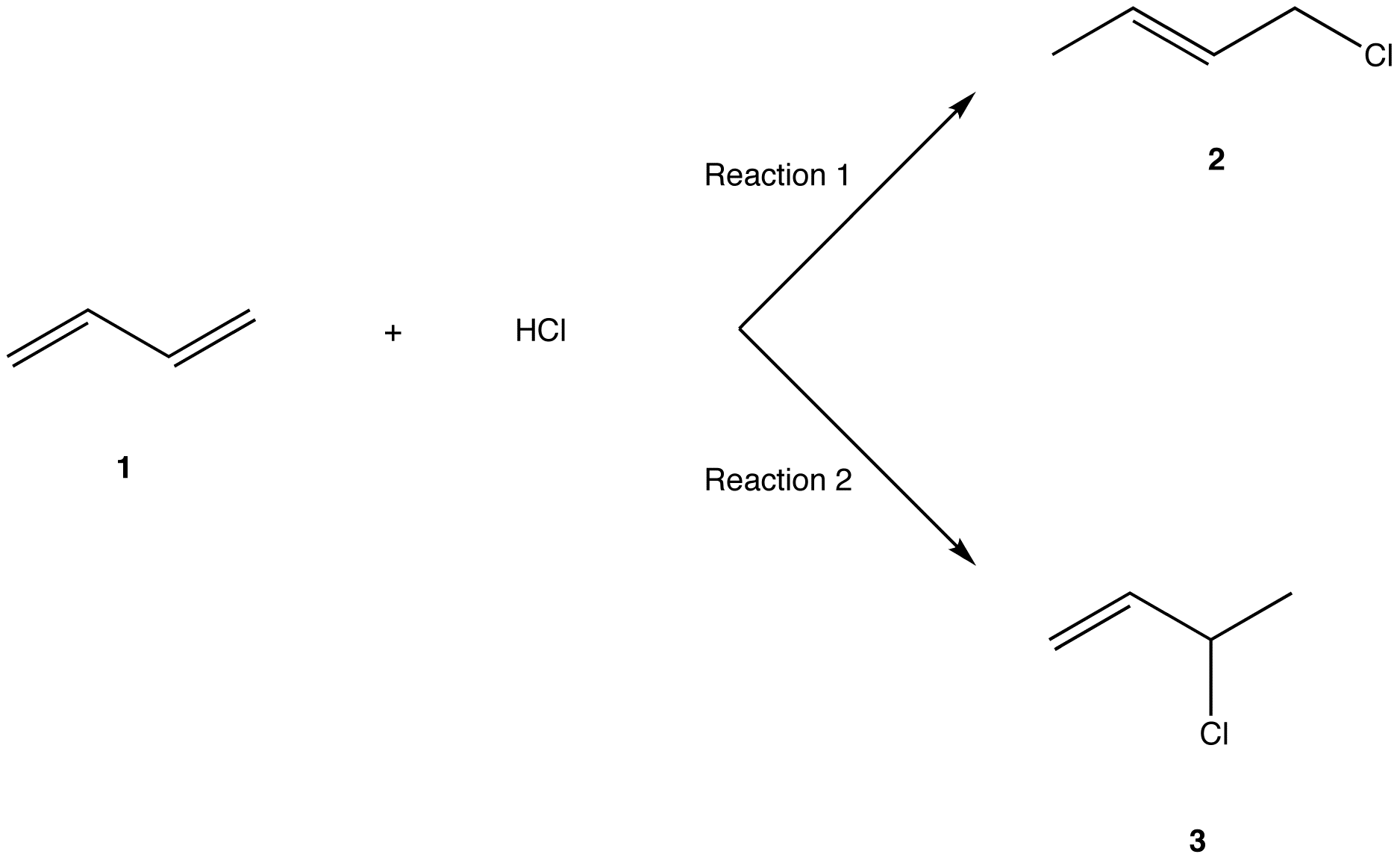
In reaction 1 the net reaction is addition of a hydrogen atom to c 1 and a chlorine atom to c 4 in 1.
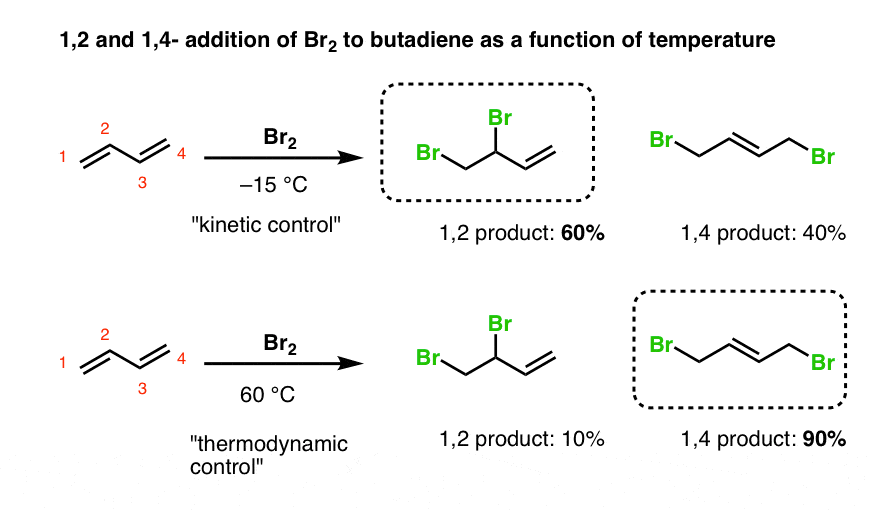
1 2 and 1 4 addition reaction. The effect of temperature. The 1 4 adduct is the thermodynamic product of the reaction since it is the more stable product. In reaction 1 the net reaction is addition of a hydrogen atom to c 1 and a chlorine atom to c 4 in 1. Skip navigation sign in.
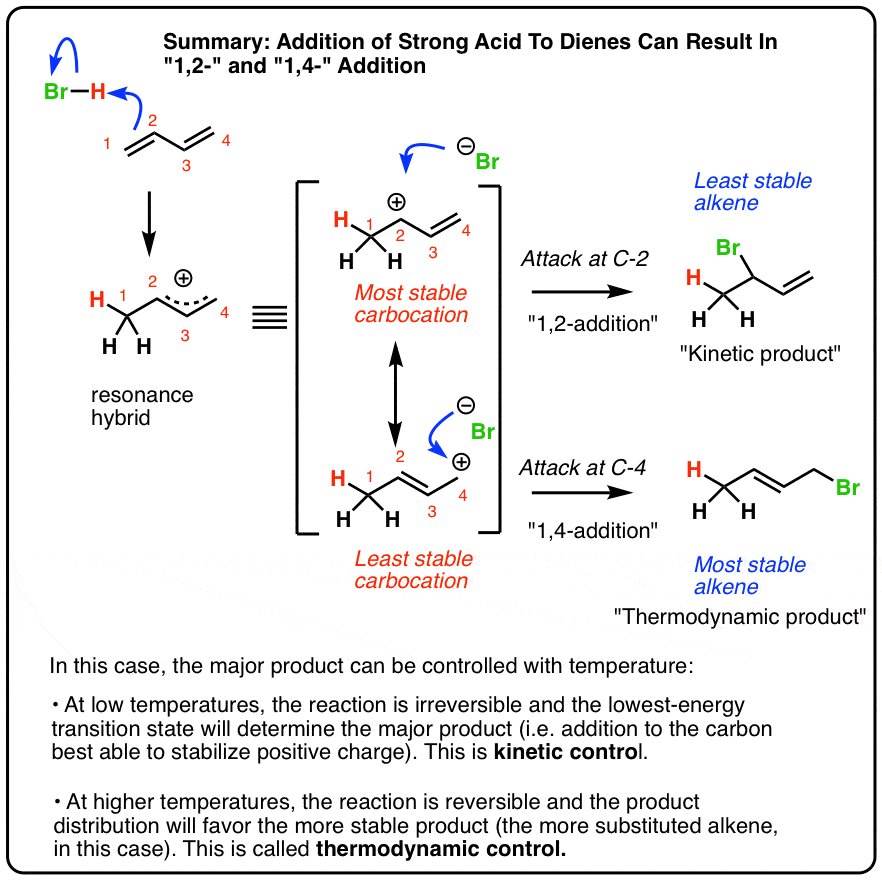
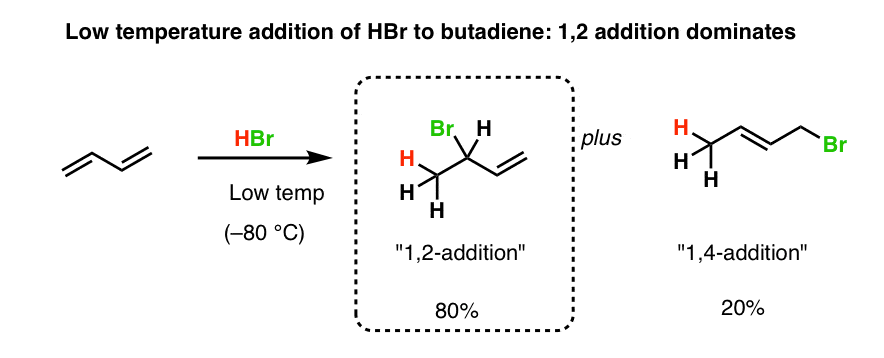
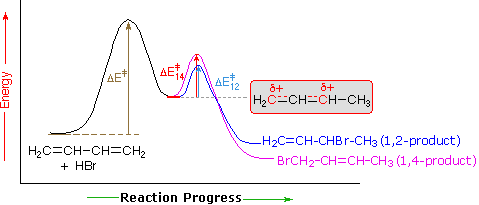
This means the competition between 1 2 and 1 4 addition is under thermodynamic control. 1 2 and 1 4 addition reaction. Points e and e represent the energies of the 1 2 and 1 4 products. For example the addition of hydrogen bromide to 1 3 butadiene at temperatures below zero leads mainly to the 1 2 addition product while addition reactions run at temperatures above 50 c with these chemicals produces mainly the 1 4 addition product.
Nucleophilic conjugate addition is a type of organic reaction ordinary nucleophilic additions or 1 2 nucleophilic additions deal mostly with additions to carbonyl compounds. The 1 2 addition starts predominating because of the proximity effect. They are formed by a 1 4 and 1 2 addtion respectively. Hence reaction 1 is called 1 4 addition and its product 2 1 4 adduct.
The regiochemistry changes when the reaction is carried out at lower temperatures. The bases used in the reaction include. Hence the activation energies for the forward reactions is equal to the difference in energy between d and c for 1 2 addition and d and c for 1 4 addition. Two electrophilic addition reactions could occur between 1 3 butadiene 1 and hydrogen chloride.
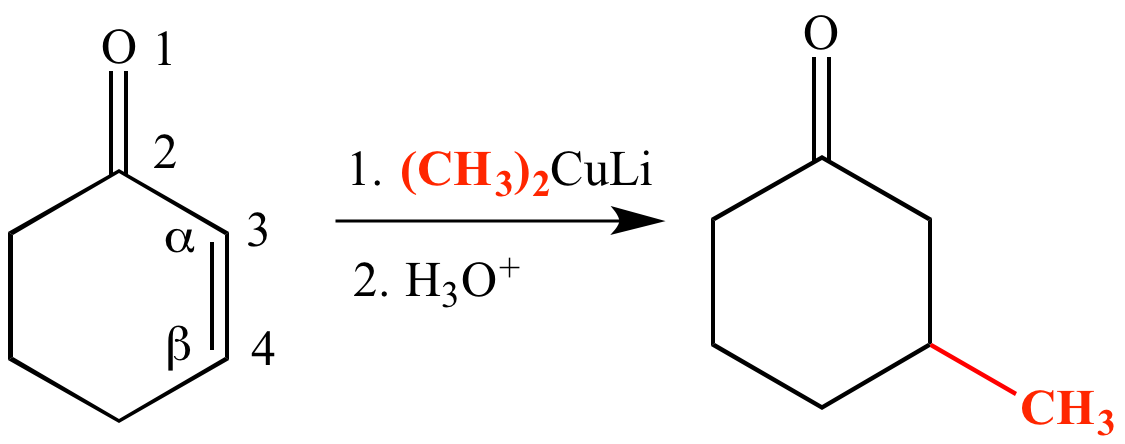
Simple alkene compounds do not show 1 2 reactivity due to lack of polarity unless the alkene is activated with special substituents with α β unsaturated carbonyl compounds such as cyclohexenone it can be deduced from. 1 4 addition is an electrophilic addition reaction of conjugate dienes. Whether more 1 2 addition or 1 4 addition product is created depends largely on the temperature at which the reaction is run. Two electrophilic addition reactions could occur between 1 3 butadiene 1 and hydrogen chloride.
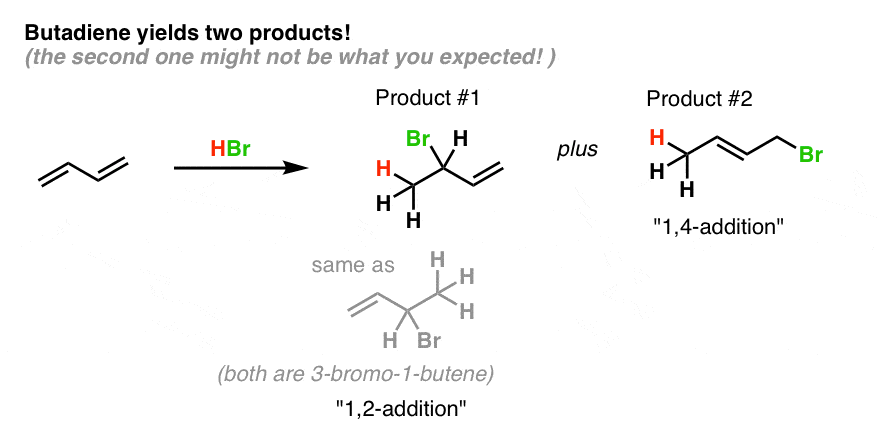
Attack of the nucleophile can occur at two different positions. 1 2 and 1 4 products in addition of br 2 to butadiene. Attack at c 4 provides the 1 4 product. This video is unavailable.
Michael addition is a thermodynamically controlled conjugate 1 4 addition reaction and competes with kinetically controlled 1 2 addition to c o. Hence reaction 1 is called 1 4 addition and its product 2 1 4 adduct. If the nucleophile is a weak base such as alcohols or amines then the 1 2 addition is usually reversible. 1 2 and 1 4 addition reaction.
When a dihalogen such as br 2 is added to a diene such as butadiene a bromonium ion intermediate will form on one of the double bonds. Since 1 2 additions to the carbonyl group are fast we would expect to find a predominance of 1 2 products from these reactions. We saw that 1 2 addition has a lower activation energy. At low temperatures 1 2 additon occurs predominantly.
Alkoxides lda naoh koh etc in protic solvents like alcohols.